Titanium catalysis enables stereoselective synthesis of C-glycosides and glycopeptides
KOH Ming Joo (Group Leader, Chemistry) October 13, 2021NUS chemists have conceived a new strategy to synthesize medicinally important C-alkyl and C-alkenyl glycosides through a titanium-catalysed reductive transformation process that reacts readily with glycosyl chlorides and various activated alkenes or alkynes.
C-Alkyl glycosides occur widely in nature and exhibit a myriad of desirable biological activities. Furthermore, the robustness of C-glycosidic bonds towards hydrolytic enzymes in vivo enables C-alkyl glycosides to play a crucial role in the design of sugar-based pharmaceutical candidates. In particular, the synthesis of C-alkyl glycosides which are conjugated to amino acids or peptide derivatives offers a powerful platform to develop sugar-based peptidomimetics. Such C-glycosylated peptide analogues are useful in drug development and biological studies to investigate the mechanism of blood-brain transport involving bioactive peptides as well as the role of glycosylation in peptide stabilisation. Unfortunately, existing non-catalytic protocols often rely on superstoichiometric amounts of reagents which limit practicality, whereas current catalytic protocols exhibit limited scope that hamper applicability to drug and glycopeptide preparation.
A research team led by Prof KOH Ming Joo, from the Department of Chemistry, National University of Singapore has developed a reaction that promotes carbon-carbon bond formation between glycosyl chloride donors and electron-withdrawing alkenes/alkynes under mild reductive conditions to give stereodefined C-glycosides (see Figure 1). Mechanistic studies revealed that the titanium catalytic species accelerate the generation of glycosyl radical intermediates and their addition across the p-bond. Equally crucial is the use of the triethylamine hydrochloride proton source, which allows for efficient protonolysis (cleavage of a chemical bond by acids) to turn over the catalytic cycle. Further insights were obtained from Density Functional Theory (DFT) calculations performed by Dr ZHANG Xinglong, a collaborator from the Institute of High Performance Computing at the Agency for Science, Technology and Research (A*STAR).
Prof Koh said, “Our new titanium-catalysed manifold is not only an advancement in the field of catalytic glycosyl radical functionalisation, but also contributes significantly to carbohydrate research by providing an enabling avenue to access high-value C-glycoside building blocks.”
“We expect our developed protocol to facilitate biological studies and fuel new medicinal chemistry initiatives towards the development of glyco-based therapeutic candidates. Furthermore, the mechanistic insights derived from our work are likely to inspire future efforts in the design of new stereoselective transformations to access other important classes of carbohydrate compounds,” added Prof Koh.
The research team plans to employ this work to the synthesis of a library of C-glycosides for potential biological testing.
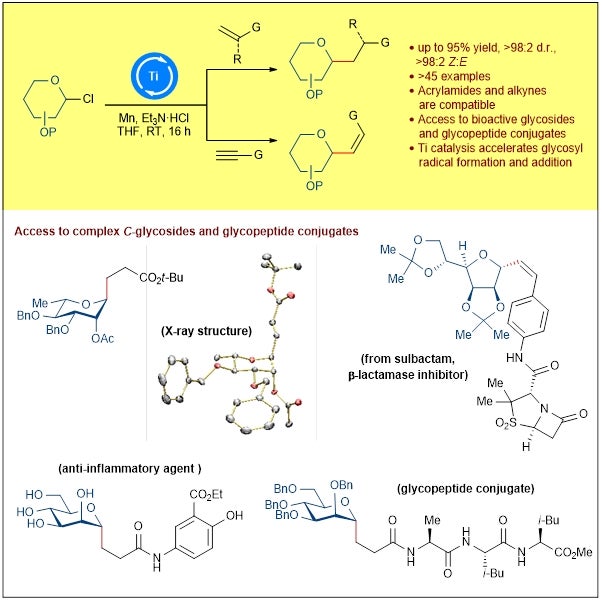
Figure 1: Schematic showing the development of a general titanium-catalysed glycosyl radical functionalisation regime that offers expeditious access to valuable C-alkyl and C-alkenyl glycosides. [Credit: Chem]
Reference
Jiang Y; Wang Q*; Zhang X*; Koh MJ*, “Synthesis of C-glycosides by Ti-catalyzed stereoselective glycosyl radical functionalization” CHEM DOI: 10.1016/j.chempr.2021.09.008 Published: 2021.


