Designing medicated tablets that can be consumed without water
Celine LIEW (Group Leader, Pharmacy) () January 22, 201522 Jan 2015 NUS researchers successfully explored how sparing and effective use of disintegrant combinations can help to enhance fast disintegration of tablets.
Oral tablets are the most common form for formulated medicines today and they are typically swallowed intact with the aid of water. However, some patients, particularly children and the elderly, find it difficult to swallow whole tablets. As such, there is growing interest in the pharmaceutical industry to design orally disintegrating tablets that breakup very rapidly into smaller fragments in the mouth on contact with saliva to ease swallowing. Moreover, such tablets can be consumed without water and are also useful for those on the move. Disintegrants are additives added in the tablet formulation to facilitate tablet breakup after administration. Their efficient use in tablet formulations is critical functionally but for a cost-effective and good quality product, a low but effective concentration is needed. Although it is widely accepted that different classes of disintegrants differ in how they cause tablets to breakup, the possibility of improving tablet disintegration by mixing functionally different disintegrants has not been investigated extensively and systematically to date (see Figure).
A team led by Prof Celine LIEW including Dr Parind DESAI and Mr Patrick ER, and in collaboration with Prof Paul WS HENG at GEA-NUS Pharmaceutical Processing Research Laboratory, Department of Pharmacy, investigated the effects of disintegrant combinations on disintegratability of tablets by a Quality by Design approach. The study findings underpinned the importance of optimizing for the “right”, synergistic combinations of disintegrants alongside specific formulation and processing parameters to enable fast tablet disintegration. The developed fast disintegrating formulations employing sparing and effective use of disintegrant combinations show promise for producing good quality yet cost-effective orally disintegrating tablets using conventional tableting technologies.
This study was well-received by the industry. In recognition for excellence in excipient research work at graduate level, Dr Parind Desai was awarded the International Pharmaceutical Excipient Council – Americas (IPEC-Americas) Foundation Graduate Student Scholarship Award in 2014 by IPEC-Americas, a global federation promoting quality in pharmaceutical excipients. He was also the recipient of the Best Graduate Researcher Award (Pharmacy) – 2014.
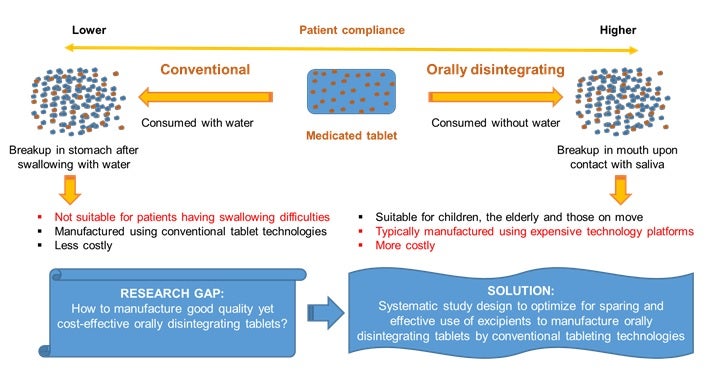
Figure illustrating the rationale for designing medicated tablets to be consumed without water. [Image credit: Parind DESAI]
Reference
Desai PM, Er PXH, Liew CV, Heng PWS. “Functionality of disintegrants and their mixtures in enabling fast disintegration of tablets by a Quality by Design approach” AAPS PharmSciTech. 15 (2014) 1093.


