Photosynthesis with water and air
February 28, 2024National University of Singapore (NUS) researchers have developed a microporous covalent organic framework with dense donor–acceptor lattices and engineered linkages for the efficient and clean production of hydrogen peroxide (H2O2) through the photosynthesis process with water and air.
Traditional industrial production of H2O2 via the anthraquinone process using hydrogen and oxygen, is highly energy-intensive. This approach employs toxic solvents and expensive noble-metal catalysts, and generates substantial waste from side reactions. In contrast, photocatalytic production of H2O2 from oxygen and water offers an energy-efficient, mild and clean route. Most importantly, it addresses the common drawbacks of existing photocatalytic systems, such as low activity, heavy use of additional alcohol sacrificial donors, and the necessity for pure oxygen gas input.
A research team led by Professor Donglin JIANG from the Department of Chemistry, NUS has developed a new type of photocatalyst for the efficient artificial photosynthesis of H2O2 from water and air. The researchers constructed hexavalent covalent organic frameworks (COFs), in which the skeleton is designed to be donor-acceptor π columns for high-rate photo-induced charge generation and catalytic active sites. In parallel, the pore is engineered with hydraulically sensitive trigonal microporous channels for immediate delivery of reactants (water and oxygen) (see Figure). As a result, these hexavalent COFs produce H2O2 spontaneously and efficiently from water and atmospheric air when exposed to visible light in both batch and flow reactors. Under laboratory conditions, the COFs demonstrate a quantum efficiency of 17.5% under visible light at 420 nm in batch reactors. This system can be developed to construct self-cleaning surfaces and for disinfection treatments.
The research findings were published in the journal Nature Catalysis.
Prof Jiang said, “In this work, we successfully addressed a key and common issue in photocatalysts, electrocatalysts and heterogeneous catalysts, which is the efficient supply of charges and mass to catalytic sites. Our focus on precise structural design at the atomic level to explore both the skeletons and pores of COFs has led to the creation of an artificial photosynthesis system for H2O2 production, achieving unprecedented photocatalytic efficiency.”
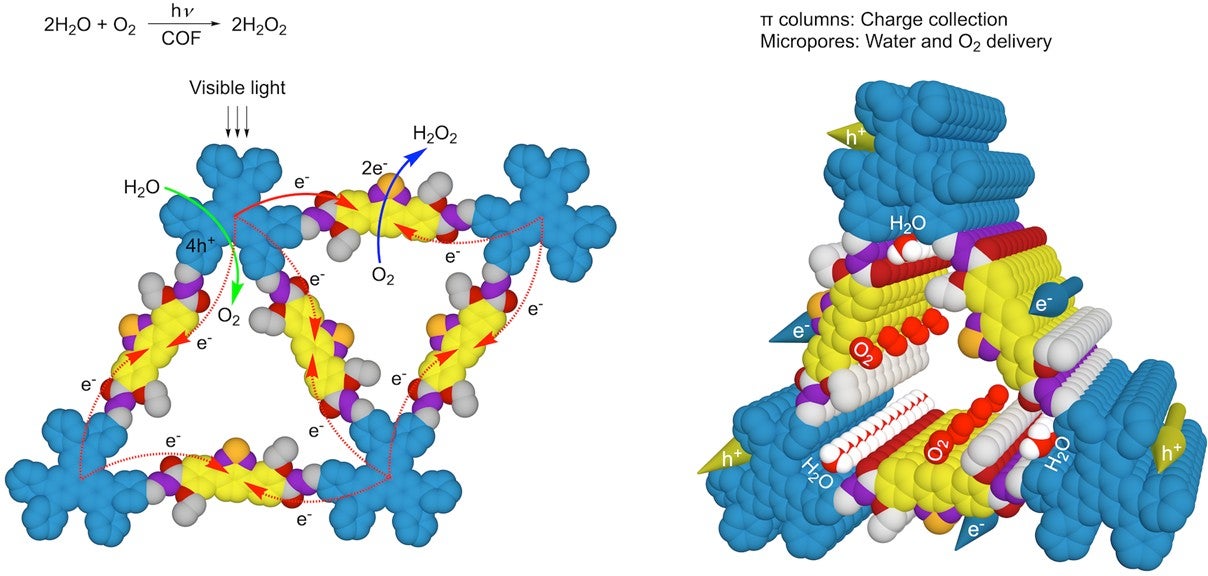
The illustration shows a newly designed hexavalent covalent organic framework (COF) material that mimics photosynthesis.
(Left figure) Light triggers the transfer of an electron from a donor site to an acceptor site within the material (indicated by red arrows). This process transfers four positive charges to the donor site, which are then used to split water molecules into oxygen (indicated by green arrows). At the acceptor site, two electrons combine with oxygen to produce hydrogen peroxide (indicated by blue arrow).
(Right figure) The structure of the material allows for efficient movement of electrons (shown in yellow), positive charges (shown in blue), water, and oxygen throughout the single layer. This material has the potential to convert light energy into chemical energy in a similar way to natural photosynthesis. [Credit: Nature Catalysis]
Reference
Liu R.; Chen Y.; Yu H; Položij M; Guo Y; Sum TC; Heine T; Jiang D*, “Linkage-engineered donor–acceptor covalent organic frameworks for optimal photosynthesis of hydrogen peroxide from water and air” Nature Catalysis DOI: 10.1038/s41929-023-01102-3 Published: 2024.


