Genome mining leads to a new type of peptide prenylation
Brandon MORINAKA (Group Leader, Pharmacy) May 19, 2021Researchers from NUS used a genome mining approach to identify a new cyclic peptide (tolypamide) derived from a cyanobacterium. The biosynthesis of tolypamide features a new type of enzyme that is able to prenylate a range of substrates and has potential use in pharmaceutical chemistry and synthetic biology.
Bioactive peptides have secured a stronghold in the pharmaceutical market due to the numerous advantages such as effective targeting of protein-protein interactions, low off-target effects, and low toxicity. These advantages are often compromised due to poor bioavailability and cell-permeability. One way to improve the membrane permeability is to increase the lipophilicity by appending an alkyl chain to the molecule (prenylation). These transformations, which can be catalysed by enzymes involved in the biosynthesis of cyanobactin natural products, can potentially boost the therapeutic efficacy of unmodified peptides. Also, cyanobactin enzymes have broad substrate selectivity and act in a highly selective and specific manner with regards to the position and orientation of the amino acid residues. Understanding the rationale behind the selectivity and specificity of these catalysts will help harness the ability of these enzymes as biochemical tool kits.
In collaboration with an international research team, Prof Brandon MORINAKA from the Department of Pharmacy, National University of Singapore discovered a new type of cyanobactin enzyme known as ToIF. It exhibits specific selectivity for the modification of serine and threonine residues in peptides. This work is a joint research effort with Prof Eric SCHMIDT from the University of Utah and Dr Muriel GUGGER from the Institut Pasteur. Proteins from a subfamily of prenyltransferases were mapped into a sequence similarity network. The researchers applied targeted genome mining to analyse the gene clusters encoding these proteins and identified ToIF enzyme and the tol biosynthetic pathway. They found that the TolF enzyme is involved in the production of tolypamide which has a hydrophobic prenyl functional group attached to its structure (Figure 1). Such molecular attachments are useful for improving the membrane permeability of therapeutic compounds. The TolF enzyme was functionally validated by in vitro biosynthesis of the tolypamide natural product (Figure 2). Additional biochemical assays show that the TolF enzyme is also able to facilitate forward prenylation on the threonine and serine residues on various synthetic peptides.
To date, the only other member of this class of enzymes (prenyltransferase) identified to act on serine or threonine residues is TruF1. However, its poor solubility and low in vitro activity has prevented extensive characterisation. The discovery of TolF provides an opportunity to study the structural biology and evolution of this enzyme family.
Prof Morinaka said, “Further characterisation of the TolF enzyme will enable a more complete understanding of substrate specificity and selectivity of prenyltransferases. This will facilitate the rational engineering of these biocatalysts as powerful tools in the field of synthetic biology.”
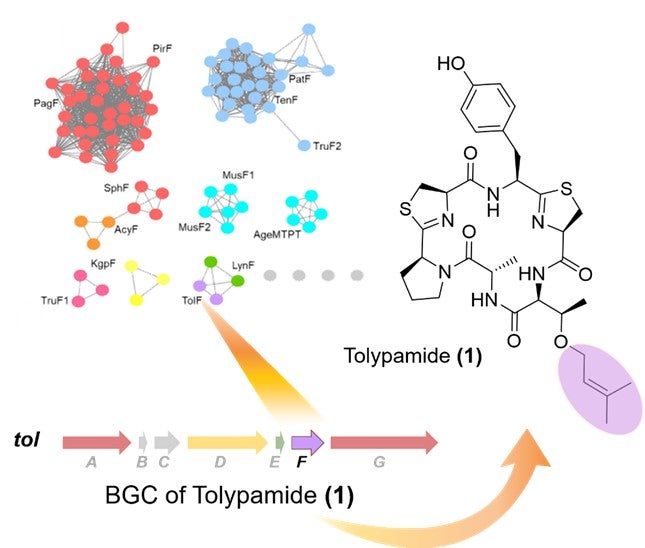
Figure 1: Targeted genome mining of prenyltransferases identified the TolF enzyme and the tol biosynthetic gene cluster (BGC). The top left shows a protein sequence similarity network of prenyltransferases. The biosynthetic gene cluster for the tol pathway is shown and was linked to the biosynthesis of tolypamide. Tolypamide contains a forward prenylated threonine residue (coloured in purple).
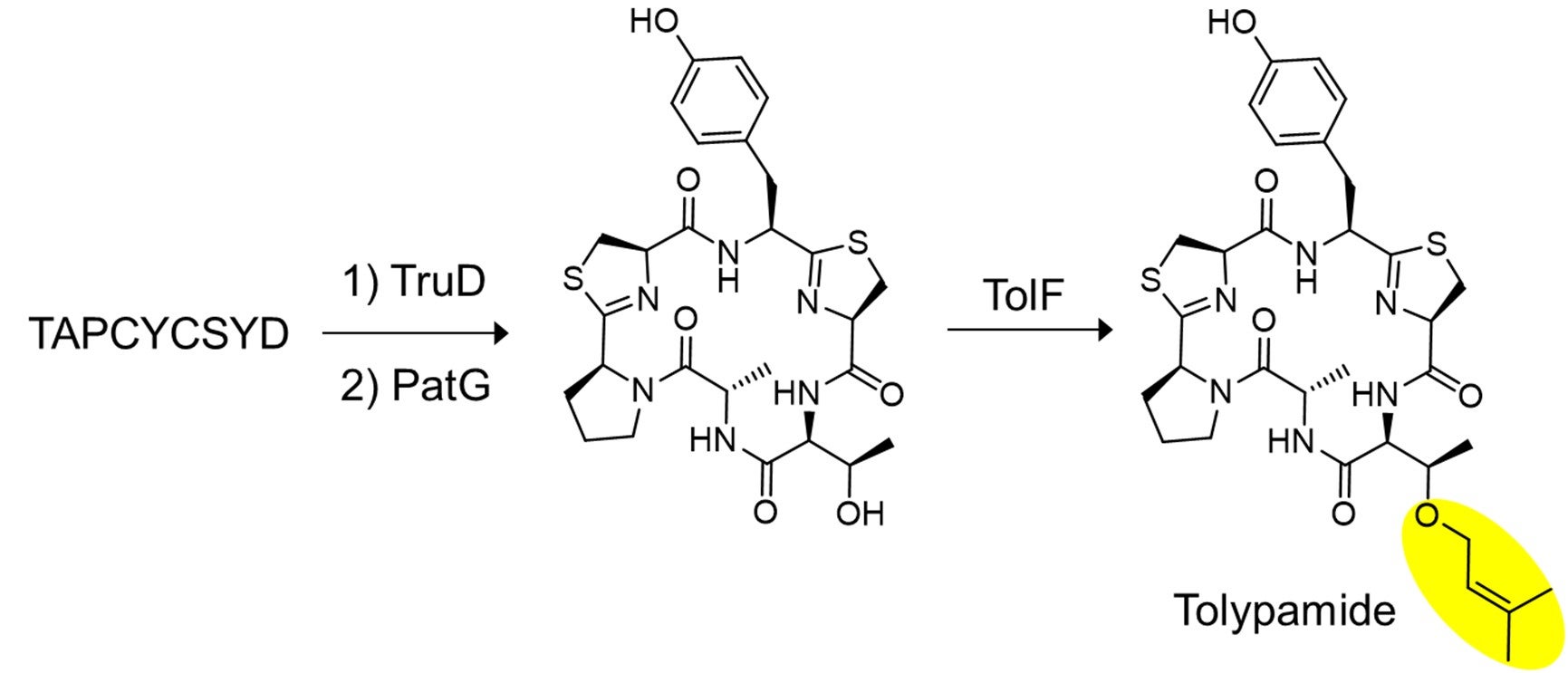
Figure 2: In vitro biosynthesis of tolypamide and functional validation of TolF. A key set of experiments were carried out to show that TolF acts on the heterocyclized cyclic peptide. The synthesized peptide, TAPCYCSYD was sequentially incubated with the enzymes, TruD (heterocyclase) and PatG (macrocyclase) to provide the heterocyclized cyclic peptide. Incubation with TolF gave quantitative conversion to the natural product tolypamide.
Reference
Purushothaman M; Sarkar S; Morita M; Gugger M; Schmidt EW*; Morinaka BI*, “Genome-Mining-Based Discovery of the Cyclic Peptide Tolypamide and TolF, a Ser/Thr Forward O-Prenyltransferase” ANGEWANDTE CHEMIE-INTERNATIONAL EDITION Volume: 60 Issue: 15 Pages: 8460-8465 DOI: 10.1002/anie.202015975 Published: 2021.


